Introduction
Facial schwannomas are benign tumors originating from the facial nerve and can develop at any location along its pathway. Managing these tumors can present medical complexities, as the potential risks of treatment may sometimes outweigh those associated with the tumor's natural development.1) Most tumors in the parotid gland are not of neurogenic origin. Tumors originating from the facial nerve account for about 0.2 to 1.5 percent of all tumors occurring in the parotid gland.2) The primary presenting symptom often involves the painless appearance of a mass within the parotid gland. These symptoms can encompass a broad spectrum, ranging from an asymptomatic mass to a painful one in the parotid region that concurrently affects the facial nerve.3)
Various diagnostic methods like imaging tests, fine needle aspiration cytology (FNAC), core needle biopsy (CNB), and intraoperative frozen biopsy have been used to make accurate diagnosis.4) FNAC is generally recommended for evaluating the parotid gland tumor, although the diagnostic accuracy can vary widely between 79% and 97%.4) However, it is impractical for the diagnosis of intraparotid facial nerve schwannoma (IPFNS). These inconclusive findings usually do not eliminate the need for surgical intervention to obtain a more substantial tissue sample for histological analysis.5) Due to its rarity and the lack of distinct clinical and radiological markers, it is often challenging to diagnose IPFNS before surgery.6)
Managing IPFNS with normal facial nerve function is particularly challenging. In this situation, surgeons face ethical and legal challenges in deciding between conservative management and complete surgical removal, given the risk of damaging the facial nerve during surgery.
We reported a case of recently enlarged IPFNS with normal facial nerve function which was not accurately diagnosed preoperatively. Through this case report, we reviewed the updates of diagnosis and treatment for IPFNS.
Case Report
A 42-year-old female patient visited our clinic, presenting with a painless mass below the ear. She reported that the mass had recently grown without inflammatory sign. The mass was firm and mobile with clear border, and facial expression was normal.
The contrast enhanced computed tomography (CT) findings revealed a 3.3 cm-sized poorly enhanced, encapsulated mass (Fig. 1A). FNAB indicated a few benign ductal epithelial nests only and negative for malignant cells. There were no specific findings, such as pain, during the FNAB in this patient. Differential diagnoses considered were benign tumor such as pleomorphic adenoma or low-grade salivary gland cancer. Due to its recently increasing size, we decided to perform surgery after counseling with patient.
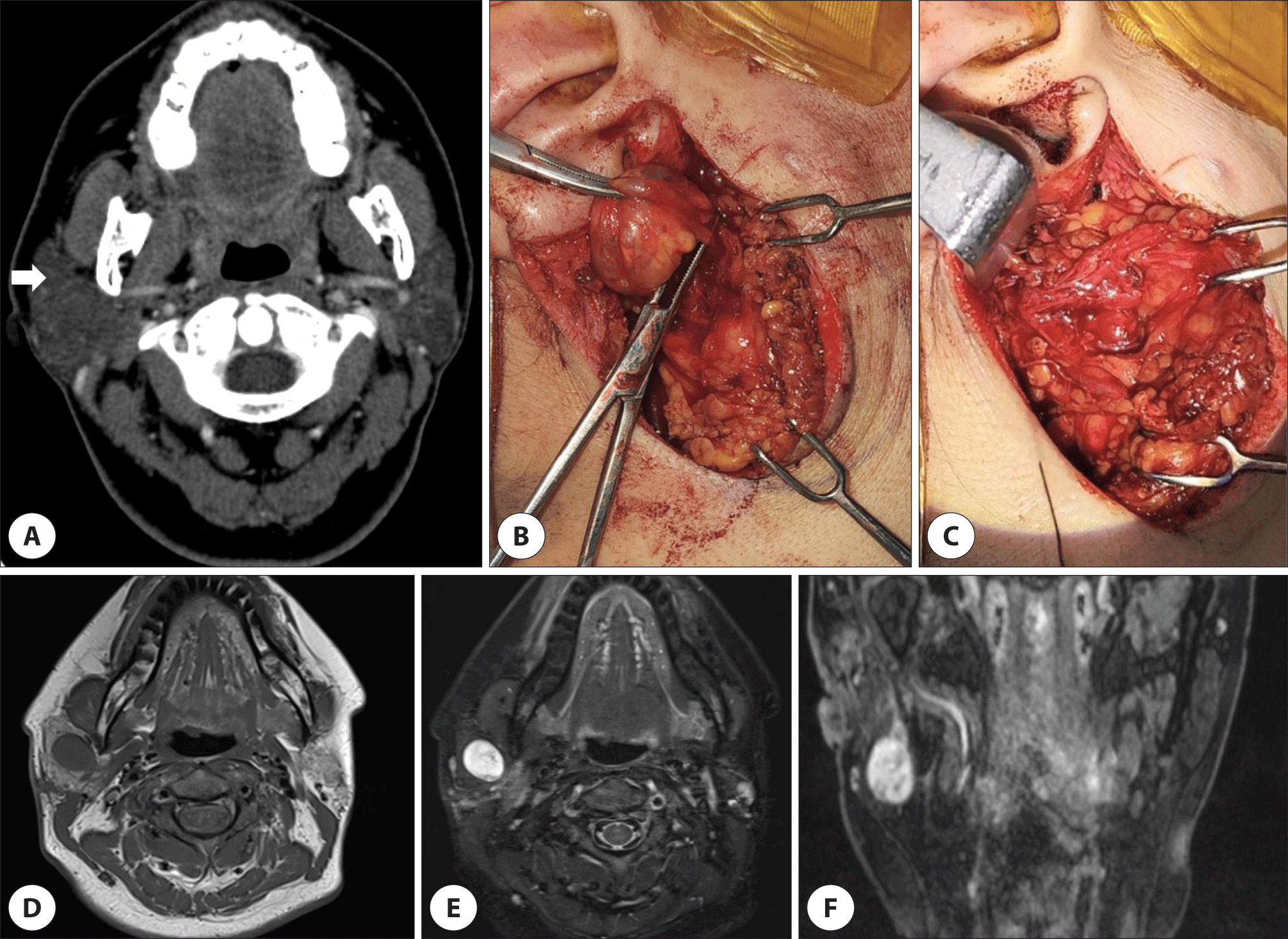
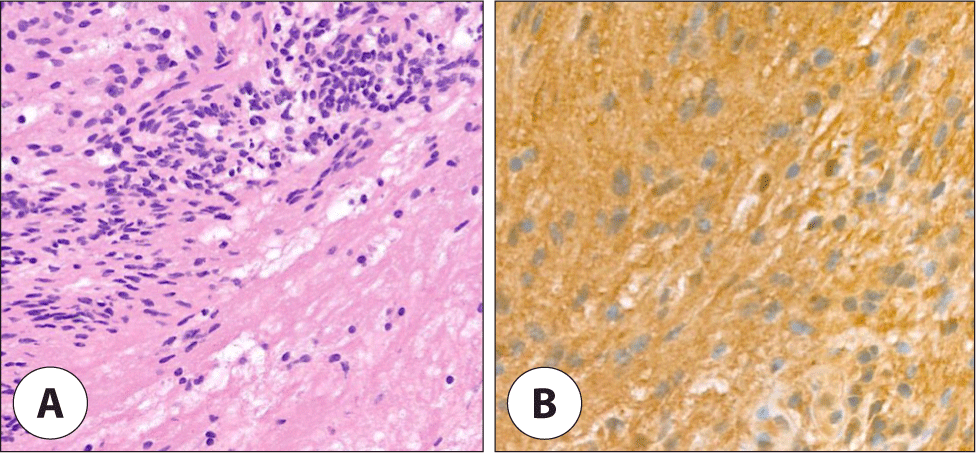
After the induction of general anesthesia, the patient was appropriately positioned, and the surgery was performed using facial nerve monitoring equipment (NIM-Response 3.0, Medtronic, Minneapolis, MN, USA). During surgery, the main trunk of facial nerve was encased by the tumor which was not separated by gentle dissection from the tumor (Fig. 1B). Additionally, when direct nerve stimulation was applied to the tumor, an electric signal was detected. The tumor was partially resected, and a frozen biopsy was sent due to suspicions of malignancy with facial nerve involvement or a nerve-origin tumor, subsequently confirmed schwannoma (Fig. 2A, B).
The tumor ramained in the deep lobe and facial nerve continuity was preserved (Fig. 1C). Nerve stimulation of the main trunk produced a very weak response, and it was decided not to remove the deep lobe mass due to the risk of losing nerve continuity. The facial paralysis at H-B grade V was shown immediately after the surgery (Fig. 3A).
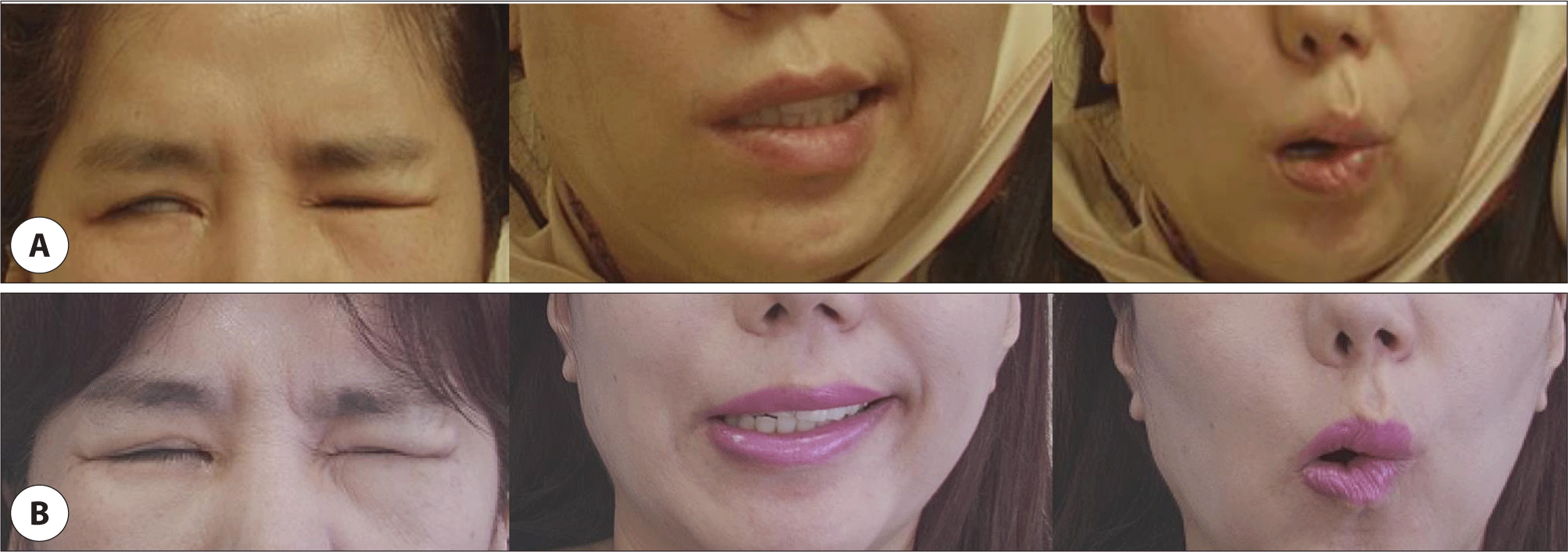
At the two weeks after the surgery, magnetic resonance imaging showed a 1.9 cm mass in the parotid deep lobe with low signal intensity on T1-weighted images and hyperintensity on T2-weighted images (Fig. 1D–F). As only the deep mass remained, it was explained to the patient that reoperation would be considered if the size increased, or facial nerve paralysis worsened. The facial paralysis improved to grade III at four months postoperatively (Fig. 3B).
The case was reviewed and approved by our Institutional Review Board (IRB approval number 2024-06-044).
Discussion
The IPFNSs are typically benign, grow slowly, and have a white, yellow, or pink encapsulated appearance, and they may exhibit signs of calcification or cystic changes.5,7) They are uncommon, accounting for less than 1% of all schwannomas, and a significant portion is situated in the intratemporal area, while only 9% are found in the extratemporal segment.3,5)
The function of the facial nerve generally remains intact in cases like these. This is because the tumor’s off-center growth applies pressure to the nerve fibers but doesn’t cause direct damage to them. In a meta-analysis, the odds ratio for facial paralysis in cases of IPFNS was 0.68 compared to schwannomas occurring in other segments of the facial nerve; however, this finding was not statistically significant.8) The soft tissue surrounding the parotid gland and its internal structure provide a path of least resistance for the tumor’s expansion, which doesn’t create significant tension or compression on the facial nerve. This, in turn, helps prevent facial nerve issues at the time of diagnosis. However, it’s worth noting that peripheral facial nerve paralysis is reported in approximately 19%–27% of Facial Nerve Schwannoma cases. Additionally, post-surgical facial nerve dysfunction is quite common due to the tumor’s proximity to the nerve fibers.2,3,5)
FNAC has been rarely documented for diagnosing schwannomas in the parotid glands.9) In review article of IPFNS, only about one-third (31.6%) who had FNAC received an accurate diagnosis before treatment.1) Only a histological analysis can provide a definitive diagnosis, and immunohistochemistry for the S100 marker is necessary to confirm the neural origin, while smooth muscle actin is used to exclude the possibility of leiomyoma.3,5) However, recent European Society for Medical Oncology (ESMO) guidelines also recommend CNB in cases where FNA yields an unclear diagnosis. In this case, had CNB been performed, it is likely that IPFNS could have been diagnosed preoperatively.10)
The “target” sign, brighter on the edges and darker in the center on T2-weighted magnetic resonance imaging (MRI) images, may hint at a neurogenic tumor. MRI can also show the “string sign,” where the mass is seen just below the stylomastoid foramen, extending into it. This sign represents the normal nerve entering or exiting in continuity with the nerve sheath tumor.6)
The 3D double-echo steady-state with water excitation (3D-DESS-WE) MRI imaging successfully predicted the tumor's relationship with the main trunk of the facial nerve in 92% of the cases (23 out of 25).11) In our case, postoperative follow-up MRI employing 3D-DESS-WE revealed a ‘string sign’ extending from the facial nerve to the deep lobe tumor. These MRI findings emphasize that preoperative suspicion is essential for detecting IPFN before surgery, underscoring its importance. However, due to the rarity of IPFNS, the application of MRI for all patients is not cost-effective. MRI should be considered when the risk of post-surgical facial nerve paralysis is elevated, such as in cases with larger tumors, tumors located in the deep lobe, or when malignancy is suspected from FNA. Additionally, MRI is advisable when diagnoses from FNA or CNB are inconclusive.12)
The treatment of IPFNS has not been established, and surveillance, partial removal, decompression, and targeted radiation therapy have been tried.7) Many authors suggest that surgery may be indicated when the House-Brackmann grade is IV or worse.13,14) Surgeons frequently employ stripping surgery techniques to carefully separate the tumor from the facial nerve, particularly at the interface between the tumor’s capsule and the nerve sheath. However, the success rate for preserving the nerve using these methods varies, ranging from 25% to 70.6%.7) An alternative approach, intracapsular microenucleation, offers a less invasive option and is conducted under microscopic guidance. This method carries a reduced risk of causing facial paralysis. The procedure commences by exposing the nerve in its typical anatomical region and then advances into the tumor-affected area without harming the bundles of nerve fibers. While intracapsular microenucleation is frequently employed for schwannomas occurring in major peripheral nerves, it has seldom been reported in the context of IPFNS. The available reports generally involve only short-term follow-up.15) Surveillance may be considered when the House-Brackmann grade is III or better, there are no other symptoms, and imaging strongly suggests IPFNS.13,14) Currently, there is no known method to predict whether IPFNS will continue to grow; during surveillance, it is important to consider that IPFNS may grow 0.4 to 2.0 mm annually.8) Furthermore, surveillance studies on IPFNS predominantly indicate a lack of progression in facial nerve paralysis. Doshi et al. monitored 12 out of 14 patients with a House-Brackmann grade of III or higher over a 13-year period, observing no clinical or radiographic progression. Similarly, McMonagle et al. tracked 20 out of 53 patients with IPFNS for eight years, reporting no deficits in facial nerve function.14)
In summary, the critical lesson from this specific case is that the likelihood of IPFNS was initially underestimated, and there was no preoperative MRI evaluation. Nevertheless, the surgical strategy was adjusted to a more conservative approach after identifying the facial nerve schwannoma through an intraoperative frozen biopsy. Postoperative rehabilitation led to an improvement in the patient’s House-Brackmann grade to III. It is anticipated that the grade will further improve, and decisions regarding future treatment will be determined by the patient’s symptoms and MRI follow-up results. Clinicians must remain vigilant for the rare yet potential presence of tumors like IPFNS.
