Introduction
Mammary analogue secretory carcinoma, a recently described carcinoma of the salivary gland, was previously categorized as acinar cell carcinoma of the salivary gland or low-grade cystadenocarcinoma not otherwise specified. In 2010, Skalova et al. identified 16 samples of salivary gland carcinoma exhibiting morphologic and immunohistochemical features akin to secretory carcinoma of the breast. All but one of these samples demonstrated a recurrent balanced chromosomal translocation, t(12;15) (p13;q25) ETV6-NTRK3, a hallmark of secretory carcinoma of the breast.1) In 2017, the World Health Organization (WHO) revised the nomenclature to Secretory Carcinoma (SC).
SCs typically occur in the salivary gland, and SC within the sinonasal cavity is exceptionally rare. In this report, we present a case of SC in the sinonasal cavity.
Case Report
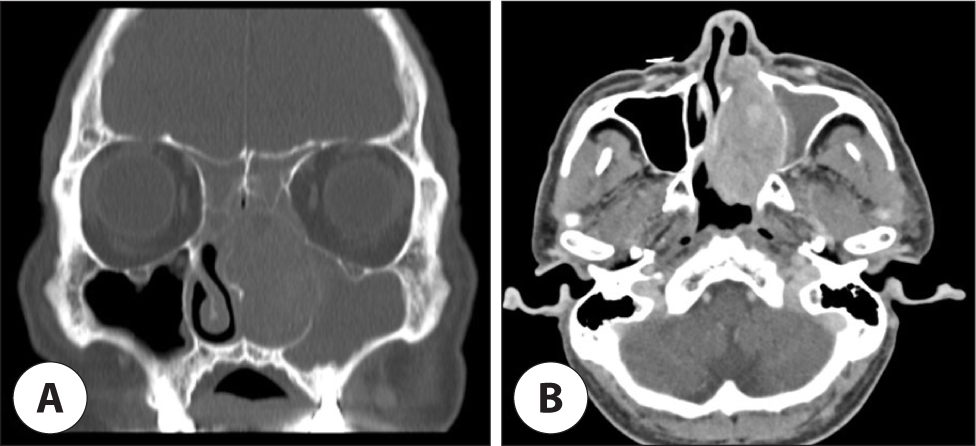
A 62-year-old male presented to the outpatient otolaryngology-head and neck surgery clinic with recurrent epistaxis from the right side. The patient had received bilateral upper molar implants and had no documented history of alcohol consumption or smoking. Endoscopic evaluation revealed a pale fragile tumorous lesion that nearly occluded the left nasal cavity and a nasal polyp in the right nasal cavity (Fig. 1). Computed tomography (CT) scans revealed a well-defined, lobulated, hypervascular mass measuring 6.4×3.3×4.0 cm with heterogeneous enhancement and internal low-density areas within the left nasal cavity. This mass obliterated the left osteomeatal unit, resulting in left maxillary, ethmoidal, and frontal sinusitis. Bony remodeling with pressure erosion of nasal septum and left maxillary medial wall was noted (Fig. 2).
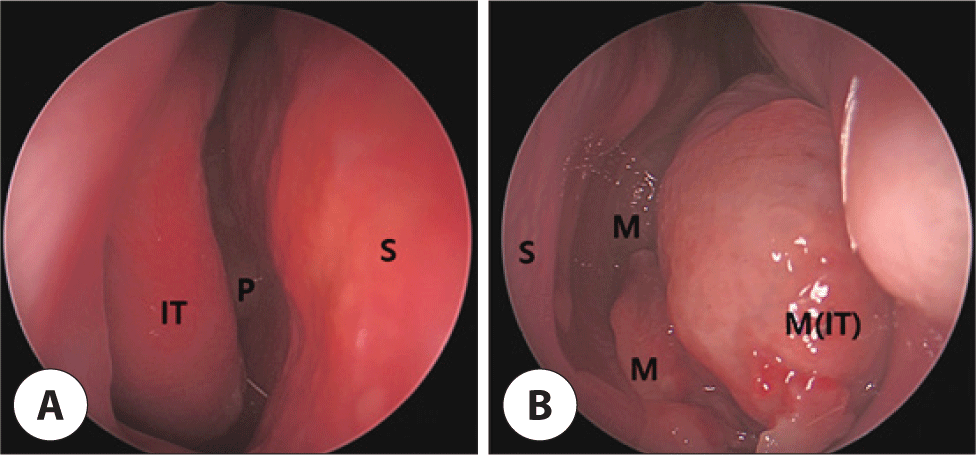
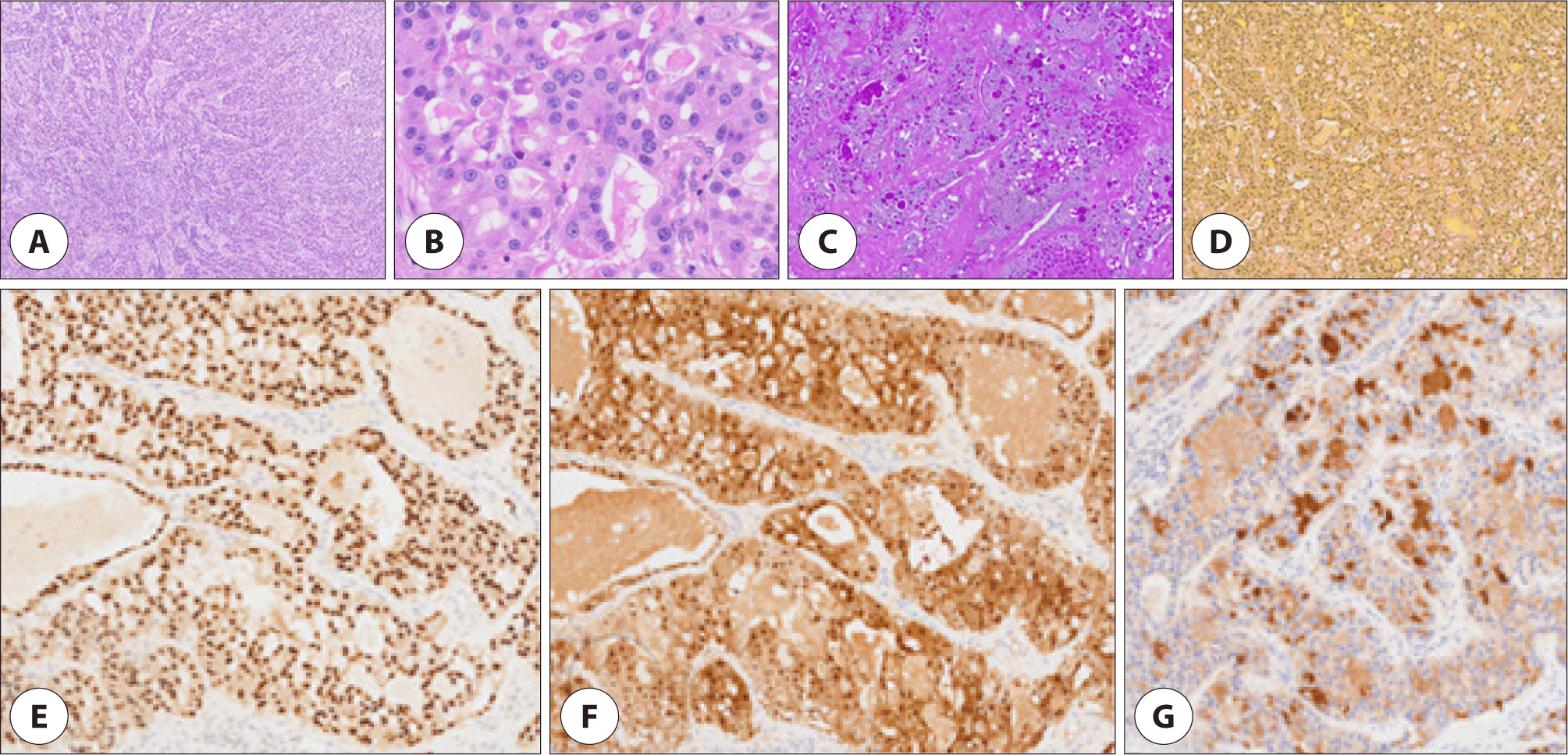
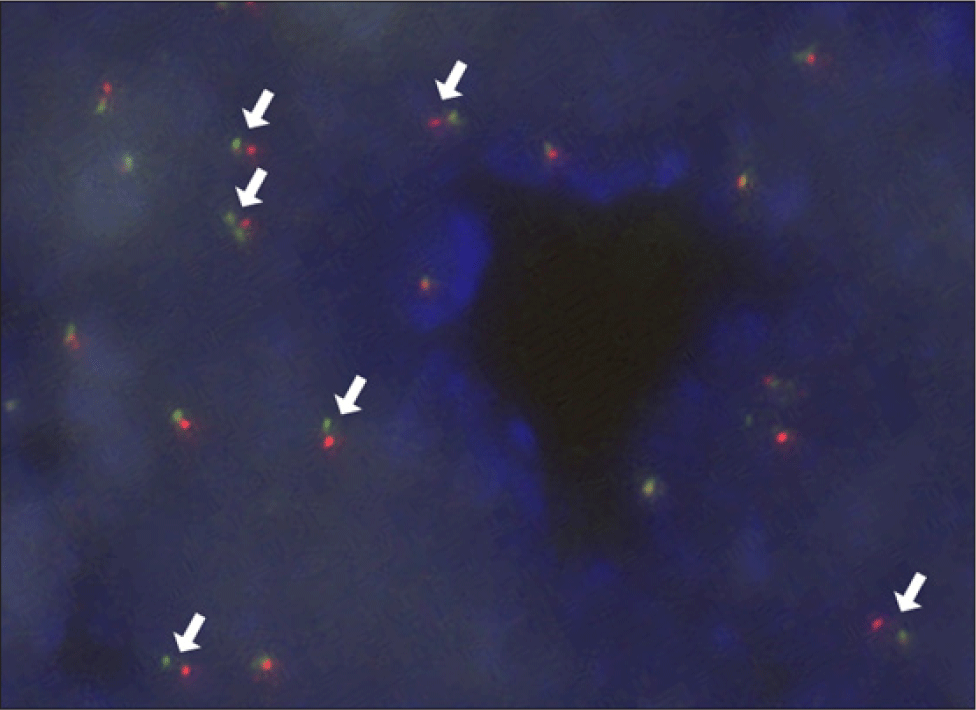
Excision of the left nasal cavity tumor, resection of the right nasal polyp, and bilateral functional endoscopic sinus surgery was performed. The tumor was lateralizing the left inferior turbinate, and upon removal, was suspected to origin from the left pterygopalatine fossa. Microscopic examination of the left nasal cavity tumor exhibited microcystic and tubular structures separated by fibrous septa. The tubular structures had abundant eosinophilic intraluminal material. Tumor cells displayed pale eosinophilic cytoplasm with granules and vacuoles. The nuclei exhibited round or oval configurations with minimal atypia, characterized by fine and granular nuclear chromatin patterns. Immunohistochemical analyses demonstrated positivity for SOX10, S-100, GCDFP-15, CEA, and CK7, and negativity for p63, panTRK, GATA3, DOG1, and MUC4. Intraluminal secretory materials exhibited positivity for mucicarmine, Periodic acid–Schiff (PAS), and D-PAS staining (Fig. 3). CD 31 showed no lymphovascular invasion. Fluorescence in situ hybridization (FISH) employing the ZytoLight SPEC ETV6 Dual Color Break Apart Probe revealed ETV-6 gene rearrangement (Fig. 4), affirming the diagnosis of SC. The right nasal polyp was identified as a nasal polyp with a retention cyst.
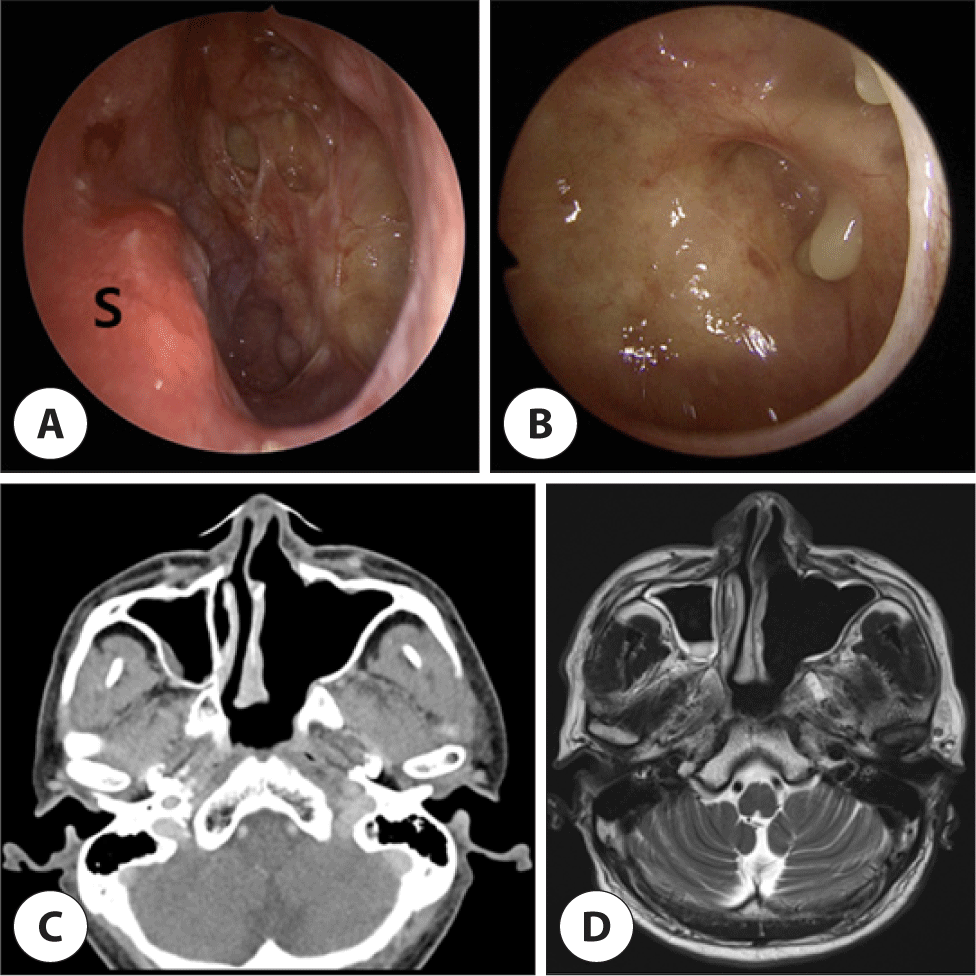
Subsequent post-operative paranasal sinus magnetic resonance imaging (MRI) and positron emission tomography-CT (PET-CT) revealed a suspicious lesion suggestive of residual tumor within the left nasal cavity. No metastasis to distant organs was discerned, and the patient was staged cT2N0M0. Following deliberation by the tumor board, surgical intervention followed by radiotherapy was planned. Two weeks following the initial surgery, left endoscopic modified medial maxillectomy was performed. Inferior turbinate, remnant middle turbinate, and medial wall of maxillary sinus were removed. The lesion suspected of harboring residual tumor on the left middle turbinate was removed en-bloc, and was confirmed as SC upon histology. Subsequently, the patient received 5400 cGy of radiotherapy. 2-year follow-up endoscopy, CT, PET-CT and MRI reported no evidence of residual tumor (Fig. 5).
Discussion
SC typically manifest in the parotid gland (>70%), submandibular gland (approximately 7%), and infrequently in the buccal mucosa, lip, soft and hard palate, minor salivary glands, base of tongue, and other sites.2) Presence of SC within the sinonasal cavity is extremely rare. SC presents as slow-growing, painless, well-circumscribed, non-encapsulated solitary mass with a characteristic white-gray hue. Lymphovascular invasion is uncommon. Histopathologically, SC can be cystic, tubular, solid, or papillary, and cytoplasm is eosinophilic with multi-vacuolation. PAS staining typically reveals bubbly secretions within the tumor. Immunohistochemical analysis consistently demonstrates positivity for CK7, CK8, CK18, S-100 protein, vimentin, mammaglobin, GCDFP-15, SOX10, and GATA3, while being negative for DOG1, p40, and p63. Most SC cases harbor the recurrent balanced chromosomal translocation t(12;15) (p13;q25) ETV6-NTRK3, but fusion of ETV6 with other genes are found on rare occasions.3-6)
Differentiating SC from other carcinoma types can pose a diagnostic challenge. Primary adenocarcinomas of the sinonasal tract are categorized into non-salivary and salivary types. Non-salivary types encompass intestinal-type adenocarcinoma (ITAC) and non-intestinal-type adenocarcinoma (non-ITAC). Non-ITACs encompass a heterogeneous group exhibiting morphologic and cytologic diversity, rendering SC susceptible to initial misclassification as non-ITAC due to shared histopathologic characteristics, including structural, cytomorphic, and immunoreactive features such as strong CK7 and S100 immunoreactivity. Features that distinguish the two include immunohistochemistry for mammaglobin, GCDFP-15 and ETV6 FISH.3) Notably, non-ITACs featuring ETV-NTRK3 fusion events have also been documented, however in those cases, non-ITACs were predominantly tubular, were arranged in back-to back pattern with scarce intervening stroma and secretory features, and showed positivity for DOG-1 and negativity or patchy positivity for S100, making the diagnosis between non-ITAC and SC challenging yet possible.7)
Overall, the prognosis of SC appears favorable. Boon et al. conducted a study involving 31 patients diagnosed with SC, reporting 5- and 10-year overall survival rates of 95% and 5- and 10-year disease-free survival rates of 89%. None of the 31 patients exhibited distant metastasis, with only one patient experiencing local recurrence. This suggests that, due to the low-grade nature of SC, prognosis is generally promising.8) However, cases of high-grade transformation in salivary gland carcinoma including SC are being reported, showing poor clinical outcome.3) Our case did not show high-grade features such as solid pattern, necrosis, or high nuclear polymorphism on histology. Meanwhile, NTRK-ETV6 fusion gene is being studied as an actionable target for curing the disease, with TRK inhibitors showing high response rates,9) making the correct diagnosis for SC clinically important as novel therapy may be applied.
To our knowledge, there have been 10 documented cases of SC within the sinonasal cavity (Table 1),3,10-15) with none reported in Korea. Aside from one case not elaborated upon in the original report and one pediatric case characterized by high-grade morphology, all cases underwent surgical resection, and six cases additionally received radiotherapy. All eight cases that underwent follow-up assessments exhibited no evidence of disease. Follow-up period ranged from 8 months to 164 months.
| Case | Age (year) and sex | Location | Size (cm) | Treatment | Outcome and follow-up (months) |
|---|---|---|---|---|---|
| Lurquin et al.10) | 67 / F | Left ethmoid sinus | NA | NA | NA |
| Xu et al.3) | 61 / M | Left maxillary sinus | 4.2 | Surgery+RT | NED / 8 |
| Baneckova et al.12) | 51 / F | Right nasal septum | 1.5×1.5×0.4 | Surgery | NED / 168 |
| Baneckova et al.12) | 65 / F | Right nasal cavity | 4×4×1.5 | Surgery+RT | NED / 48 |
| Wu et al.13) | 72 / M | Left inferior turbinate | 0.8×0.4 | Surgery | NA |
| Willis et al.11) | 39 / M | Left maxillary sinus | NA | Surgery+RT | NED / 12 |
| Yue et al.14) | 63 / M | Right maxillary sinus | 5.3×3.5×1.7 | Surgery+RT | NED / 79 |
| Yue et al.14) | 78 / F | Left nasal septum | 4.2×2.1×1 | Surgery | NED / 164 |
| Yue et al.14) | 49 / M | Right nasal cavity | 2.8×2.1×1.5 | Surgery+RT | NED / 118 |
| Cardoni et al.15) | 12 / F | Right maxillary sinus | 6.2×4.8 | Chemotherapy+surgery+RT | NED / 16 |
This case report has certain limitations. First, mammaglobin staining was not performed. WHO 5th edition criteria necessitate positivity for SOX 10, S100, and mammaglobin for the diagnosis of SC. In light of the unavailability of mammaglobin staining at our institution, GCDFP-15 staining, a useful marker in the diagnosis of breast carcinoma alongside mammaglobin, was performed and demonstrated positivity. In addition, SC typically exhibits positivity for panTRK and GATA3, both of which were negative in our case. As such, we conducted a FISH test to provide supplementary evidence of SC, which confirmed the presence of ETV6 gene translocation. Second, the follow-up duration in our case report was relatively short compared to certain previous sinonasal SC case reports. The patient is scheduled for biannual follow-up assessments, and further evaluation and management may be needed if tumor recurrence is suspected.
In summary, we have documented a case involving a 62-year-old male patient presenting with SC within the sinonasal cavity. The patient underwent surgery followed by radiotherapy, displaying no signs of recurrence after 24 months. While SC may be initially confused with non-ITAC, precise diagnosis is imperative due to its prognostic and therapeutic ramifications. Continued follow-up studies and future research endeavors on SC will contribute to a deeper understanding of this rare disease and the development of optimal treatment strategies.
