Introduction
Takayasu’s arteritis is a type of chronic inflammatory arteriopathy that occurs in the aorta and its major branches, resulting from various causes.1) In the early stages of the disease, nonspecific symptoms manifest, whereas in the later stages, several complications can occur. Complications caused by arterial stenosis, occlusion, and aneurysm formation may be accompanied by the absence of peripheral pulses, claudication, auscultatory murmurs, hypertension, Raynaud’s phenomenon, stroke, aortic regurgitation, congestive heart failure, pericarditis, and myocardial infarction.2)
Nasal septal perforation can be caused by trauma, nasal septal surgery, inflammatory diseases, tumors, systemic disorders, toxic substances, etc.3) It has also been reported to occur in patients with various rheumatic diseases. Nasal septal perforation is one of the major clinical manifestations of Takayasu’s arteritis along with Wegener’s granulomatosis and relapsing polychondritis.4) In patients with systemic lupus erythematosus, nasal septal perforation has been reported as a complication resulting from mucosal involvement, with an incidence rate of approximately 5%.5) Septal perforation has also been infrequently reported to occur in patients with juvenile idiopathic arthritis, sarcoidosis, rheumatoid arthritis, and adult-onset Still’s disease.1)
Septal perforation associated with Takayaus’s arteritis is extremely rare and has not been reported in Korea. In this context, we aimed to report a case of coexisting nasal septal perforation and Takayasu’s arteritis in a 48-year-old woman along with a literature review.
Case Report
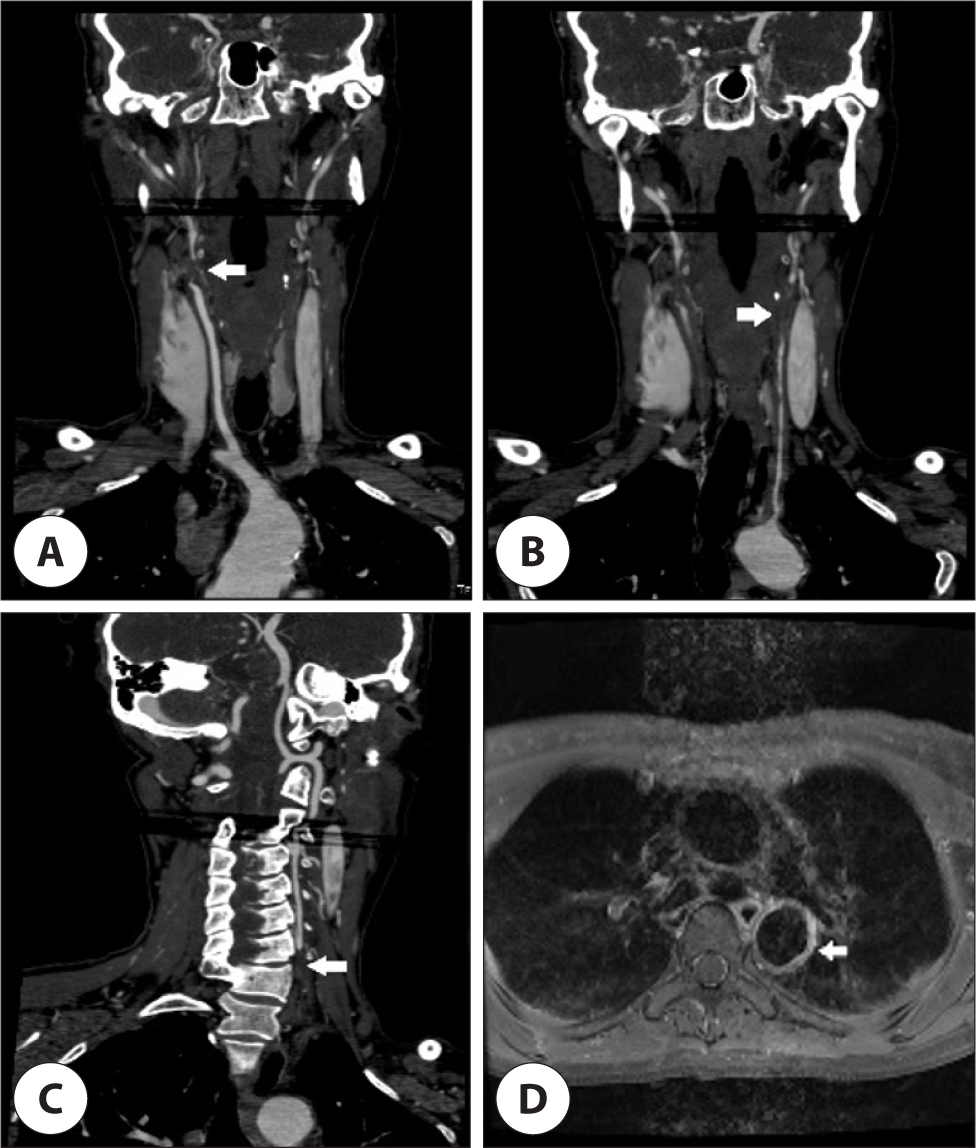
A 48-year-old previously healthy woman presented with intermittent headaches for the past 7 years. She recently experienced a significant increase in headache severity and thus sought medical evaluation. Brain magnetic resonance imaging (MRI) performed in the neurology department did not show any acute lesions. However, magnetic resonance angiography (MRA) of the brain revealed bilateral internal carotid artery occlusion and collateral blood flow in the bilateral posterior communicating arteries. Based on these findings, the patient was admitted to the neurology department of our hospital. Physical examination showed absence of pulsation in the bilateral brachial arteries, right carotid artery, bilateral radial arteries, and ulnar arteries, whereas palpable pulses were detected in the left carotid artery and bilateral dorsalis pedis. Blood pressure measurements indicated a significant discrepancy between the right (64/37 mmHg) and left (101/47 mmHg) brachial readings, as well as elevated readings in the right (164/78 mmHg) and left (178/73 mmHg) popliteal regions. Laboratory analysis revealed an elevated erythrocyte sedimentation rate and a slightly elevated C-reactive protein level of 33 mm/h and 0.22 mg/dL, respectively. According to the American College of Rheumatology guidelines, the diagnosis of Takayasu’s arteritis requires the fulfillment of at least three of the six criteria: 1) age younger than 40 years, 2) claudication of extremities, 3) decreased pulses in the upper extremities, 4) blood pressure difference of 10 mmHg or more between arms, 5) bruits over the subclavian arteries or aorta, and 6) angiographic proof of narrowing or occlusion of the aorta or its major branches. In this case, the patient met three criteria for the diagnosis of Takayasu’s arteritis: absent pulses in the bilateral upper extremities, a 40-mmHg systolic blood pressure difference between arms, and angiographic confirmation of stenosis or occlusion of the major branches of the aorta on brain MRA.6) Subsequently, the patient was referred to the rheumatology department. Further evaluation with neck computed tomography angiography indicated complete occlusion of the right brachiocephalic trunk, left common carotid artery, and left subclavian artery, as well as stenosis and occlusion of the left brachiocephalic artery. Chest MRI revealed vascular wall hypertrophy and contrast enhancement in the descending thoracic aorta and left common carotid artery suggesting arteritis (Fig. 1). Additionally, neck ultrasound examination confirmed occlusion of the right internal carotid artery and left brachiocephalic artery as well as stenosis of the left common carotid artery. To rule out autoimmune causes, further testing including rheumatoid factor, antinuclear antibodies, antineutrophil cytoplasmic antibodies, cANCA, pANCA, and anticardiolipin antibodies was conducted, all of which yielded negative results. The patient exhibited improvement in headache after initiating immunosuppressive therapy.
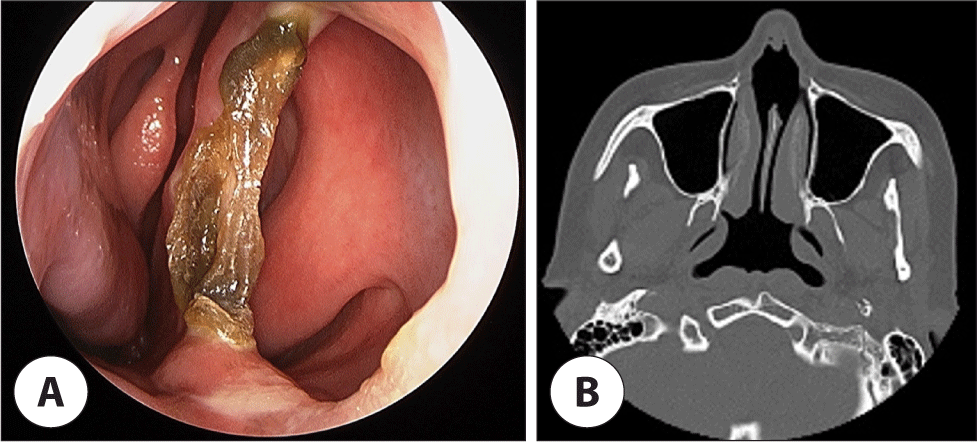
During hospitalization, an otolaryngologic consultation was requested for the management of bilateral nasal obstruction that had persisted for 1 year. The patient had no history of trauma, nasal diseases, or previous nasal surgeries as well as illicit drug use, exposure to heavy metals, or smoking. Endoscopy showed a large perforation in the caudal septum, characterized by significant dry crusts along its borders, while no other malignant, inflammatory, or granulomatous changes were observed (Fig. 2A). Paranasal sinus computed tomography imaging revealed no abnormalities in the nasal cavity or paranasal sinuses but confirmed the size of the septal perforation to be 18×14 mm (Fig. 2B). After excision of the exposed bony edges of the perforation using cutting forceps, a reduction in crust formation was observed. The patient did not experience discomfort or desire for surgical correction; therefore, conservative management was pursued.
Discussion
Takayasu’s arteritis is characterized by cellular infiltration leading to vascular tunica media thickening and localized vascular smooth muscle destruction. Fibrotic reactions and hyperplasia of the tunica media and intima occur, resulting in blood vessel narrowing or stenosis.7) In the early stages before significant vascular lumen narrowing, nonspecific symptoms are more common than ischemic ones, and during this period, patients may not be aware of the disease. In the later stages, ischemic symptoms caused by arterial occlusion occur,1) and 5%–20% of patients with Takayasu’s arteritis experience neurological symptoms such as stroke, transient ischemic attacks, and visual disturbances.8)
The treatment goal for Takayasu’s arteritis is to suppress inflammatory response and maintain stable blood flow.2) Steroids are commonly used to suppress immune response; additional immunosuppressive agents such as Methotrexate can also be utilized. Joveret et al. reported that the combination of steroids and Methotrexate reduces the total amount of steroid usage and lowers the frequency of disease relapse.9) On the other hand, studies by Hoffman et al. and Spiera et al. showed no significant difference between steroid monotherapy and combination therapy.10,11) In cases where severe symptoms persist due to arterial stenosis and occlusion even after the acute inflammatory phase, surgical interventions such as stent placement, endarterectomy, bypass graft surgery, and angioplasty may be considered.2) In the present case, steroid was administrated in the acute inflammation phase, and Aspirin, Plavix, and Methotrexate were used to manage the inflammation as a maintenance therapy.
Septal perforation occurs due to various causes, and Wegener’s granulomatosis or sarcoidosis has been reported to cause granulomatous inflammation leading to septal damage and the development of septal perforation and saddle nose deformity.12) Yamasaki reported a case of septal cartilage and maxillary sinus necrosis in a patient with Wegener’s granulomatosis and Takayasu’s arteritis.13) Additionally, septal perforation can occur due to ischemic changes. Blockage of blood flow to the septum can occur due to pressure from intranasal hemostatic agents used for controlling epistaxis or vasoconstriction caused by nasal sprays or cocaine abuse. In some rheumatic diseases, such as systemic lupus erythematosus and antiphospholipid antibody syndrome, septal perforation can occur due to ischemic changes in the septum.12,14) The septal mucosa receives blood supply from the ophthalmic arteries originating from the internal carotid artery and from branches of the maxillary artery originating from the external carotid artery, with the terminal artery of the maxillary artery, called the sphenopalatine artery, supplying the major blood flow to the septal mucosa.1) If carotid artery stenosis or occlusion occurs due to Takayasu’s arteritis, ischemic changes in the septal mucosa can lead to septal perforation. Shine et al. reported a case of saddle nose deformity in a patient with Takayasu’s arteritis,15) and Akar et al. reported the first case of septal perforation in a patient with Takayasu’s arteritis, although the patient’s condition deteriorated, resulting in death following medication treatment and cerebral vascular bypass surgery.1) In the present case, the patient had stenosis of both the internal carotid arteries, but after medication treatment, the headache symptoms improved, and the patient has been undergoing treatment without any significant complications for 11 years.
Histological biopsy to confirm granulomatous inflammation or vascular inflammation would be helpful in accurately differentiating the cause of septal perforation. However, a limitation in this case is that the patient was unable to undergo septal tissue biopsy. Nevertheless, during the 11-year follow-up period, no other systemic diseases or nasal disorders occurred aside from Takayasu’s arteritis, suggesting a low possibility of alternative causes.
Septal perforation occurring in patients with Takayasu’s arteritis has been very rarely reported, and the reported cases were associated with worsening conditions leading to death following surgery for Takayasu’s arteritis or coexistence with other rheumatic diseases. Therefore, even in patients with Takayasu’s arteritis who do not exhibit severe clinical symptoms, the possibility of septal perforation should be considered in cases where other causes have been ruled out.
